ABOUT MISCARRIAGE

Miscarriage is the most common complication during the early stages of pregnancy and occurs in about 15-25% of clinically diagnosed pregnancies, most of which in the first trimester of gestation. There are many known causes and risk factors for spontaneous abortion but about 60% of cases originate from chromosomal abnormalities1,2. Diagnosing a chromosomal abnormality as a cause of miscarriage provides important information for predicting the risk of recurrence and helps identify familial chromosomal rearrangements that can predispose couples to recurrent miscarriages or the birth of babies with congenital abnormalities and / or intellectual disabilities. In 2016, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine recommended the search for chromosomal abnormalities on the placenta, amniotic fluid and the product of conception (POC) in cases of intrauterine fetal death and perinatal mortality 3.
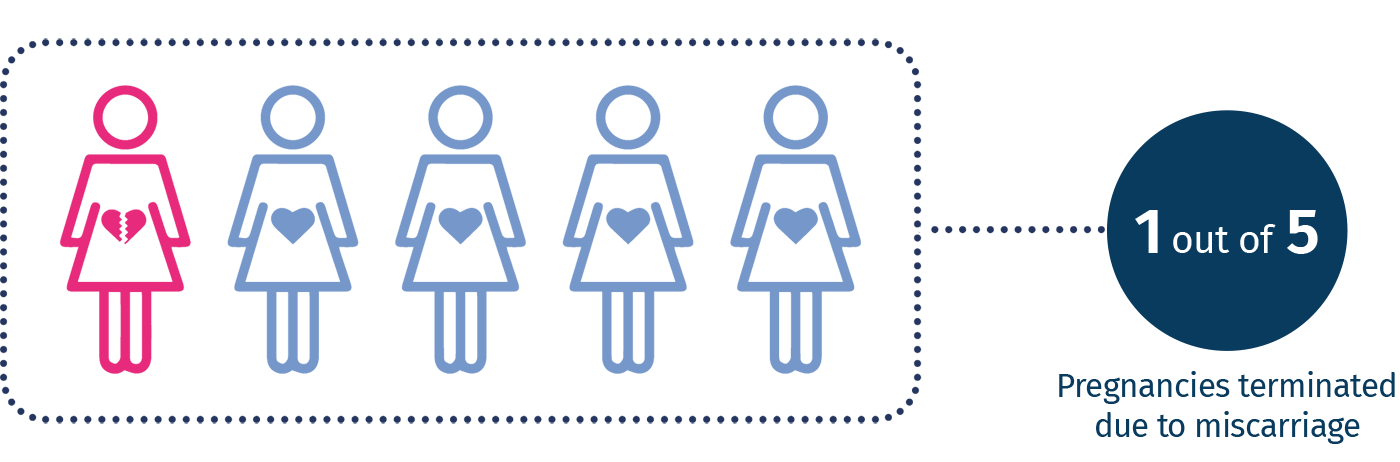
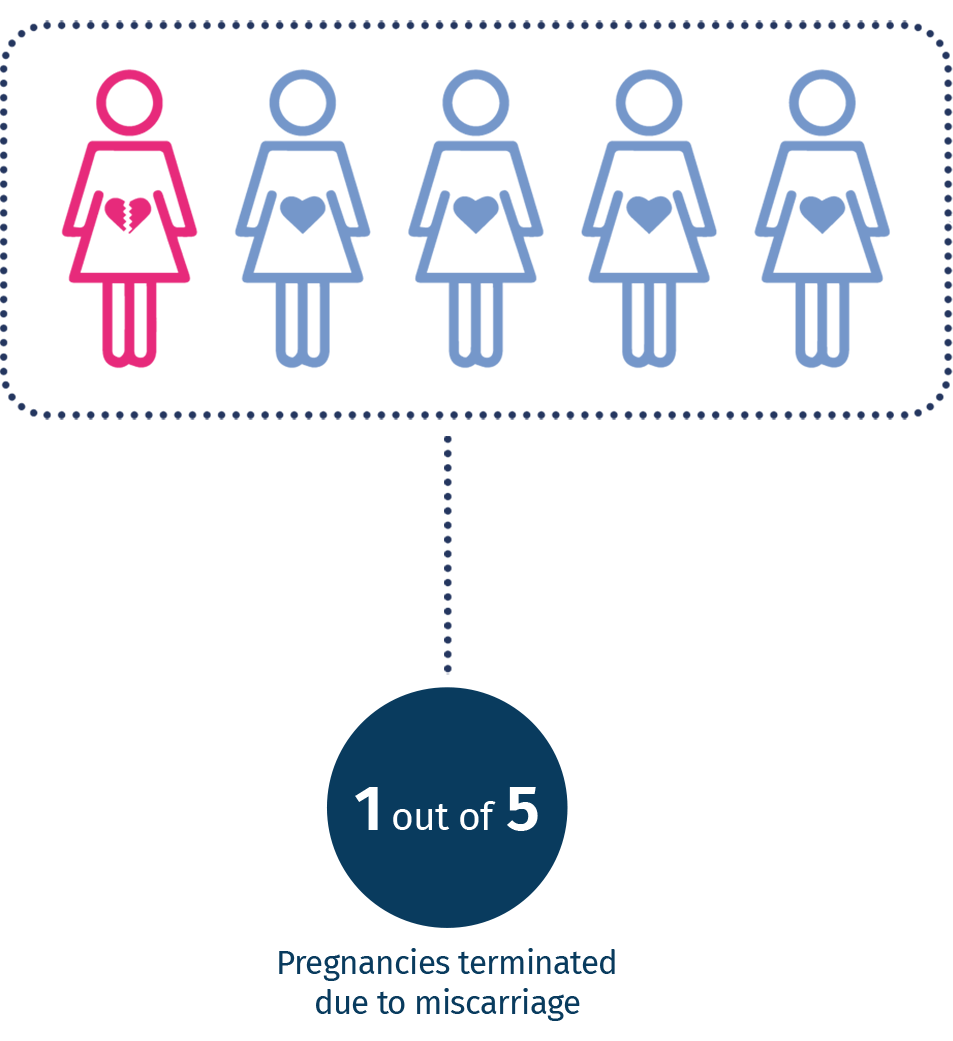
1 S. D'ippolito et al., 2017
2 JA Rosenfeld et al., 2015
3 Committee Opinion No. 581, 2016
THE FETAL KARYOTYPE BY USING TRADITIONAL CYTOGENETIC TECHNIQUES

The cytogenetic investigation (or karyotype) on the product of conception has traditionally been performed by culturing the fetal
cells present in the fetal tissue and subsequent microscopic analysis of the chromosomes in metaphase.
Traditional karyotyping from product of conception tissue is characterized by technical difficulties and diagnostic limitations
- Extended turnaround time: Cell cultures require 15-20 days for reporting of results, which are necessary for the development of colonies of fetal cells.
- Risk of culture growth failure: Sometimes it is possible that the fetal cells placed in culture do not grow adequately, resulting in the inability to achieve a diagnosis. This problem is very common; it occurs in about 50% of POC tissue cultures. The high frequency of culture failure depends on the fact that to perform a karyotype analysis with traditional techniques it is necessary to have living cells, capable of dividing once placed in culture. This is not always possible in the case of analysis on POC tissue, which contains almost all non-living cells.
- Risk of contamination with maternal cells: the POC tissue is obtained by revision of the uterine cavity, so in this sample there are also maternal cells, which could grow in cell culture, in competition with fetal cells. Therefore, when a traditional cytogenetic karyotype from abortive tissue is performed and the result is "normal female karyotype", it is not possible to rule out of having examined maternal cells instead of fetal cells, which in culture are indistinguishable.
- Resolution limits: with the cytogenetic karyotype both aneuploidies, i.e. the alterations in the number of chromosomes, responsible for the most frequent syndromes, and the structural alterations of a certain entity are clearly highlighted. In other words, structural anomalies larger than 10-15 Mb with a bandwidth resolution of 350-400 bands can be highlighted with this test. Therefore, the diagnosis of pathologies deriving from submicroscopic chromosomal alterations (microdeletions or microduplications) is missed.
- Possibility of "in vitro" artefacts: most often referable to pseudomosaicisms. This can happen in 2-3% of cell cultures.

 is an advanced molecular test that, using the latest generation of fully
automated sequencing technologies (Next Generation Sequencing - NGS), allows to determine the
fetal karyotype from the POC with high efficiency, accuracy and resolution.
is an advanced molecular test that, using the latest generation of fully
automated sequencing technologies (Next Generation Sequencing - NGS), allows to determine the
fetal karyotype from the POC with high efficiency, accuracy and resolution.
 is able to provide information on the possible causes of a miscarriage,
with the aim of helping the couple in family planning.
is able to provide information on the possible causes of a miscarriage,
with the aim of helping the couple in family planning.
TYPES OF TESTS


An advanced molecular test that, using groundbreaking sequencing technologies, screens for fetal chromosomal abnormalities in product of conception (POC) by analyzing the circulating cell-free fetal DNA (cfDNA) in maternal blood.
More Info

An advanced molecular test that, using the latest generation of fully automated sequencing technologies, determines the fetal karyotype from the product of conception (POC) tissue with high efficiency, accuracy and resolution.
More Info
Test Results


Chromosomal abnormality detected:
this result shows that the test detected a chromosomal abnormality in the fetus.

Chromosomal abnormality not detected:
this result shows the test has not abnormality. This result is suggestive of euploidy (normal fetal karyotype).

This test is unable to detect
- balanced chromosomal rearrangements (reciprocal translocations, inversions)
- chromosomal mosaicisms (i.e. the presence of two cell lines with different karyotype) with a poorly represented cell line (less than 10%)
- microdeletion/ microduplication syndromes
- structural chromosomal anomalies with a size below 6Mb
- point mutations, methylation defects, polyploidies
INDICATION FOR TESTING


SUGGESTED
- Women who have had a miscarriage


STRONGLY RECOMMENDED
- Women who have had recurrent miscarriages
- Women who have had miscarriages following IVF treatments
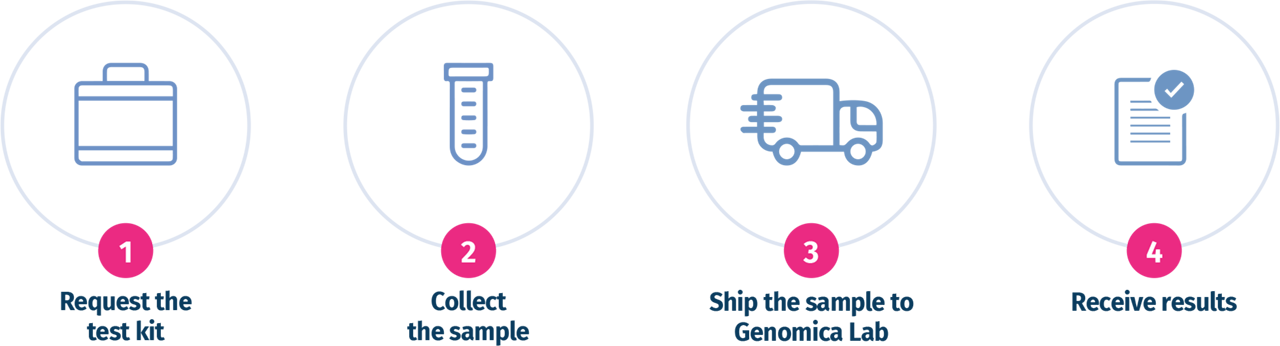
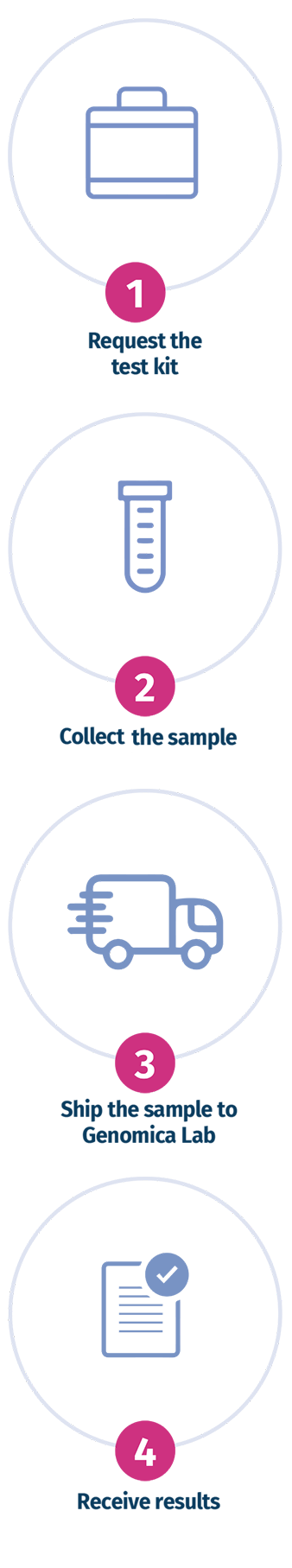
THE TESTING PROCESS

The transportation kit contains the tubes suitable for shipping the POC sample.
It is recommended to place the
sample in the supplied tube and fill it with saline solution.
The sample must be stored at +4°C until shipping.
WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000
genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000 genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience
in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
Information request for POCAdvance test

Fill out this form for a free consultation.
One of our professionals will contact you, free of charge and without obligation, to provide you with all the information you need.


 Italiano
Italiano English
English
